Explain the Difference and the Relationship Between Heat and Temperature
Heat is measured in Joules. More specifically heat is the total amount of thermal energy in a system.
Difference Between Heat And Temperature With Comparison Chart Key Differences
In cooler air the molecules are closer together.
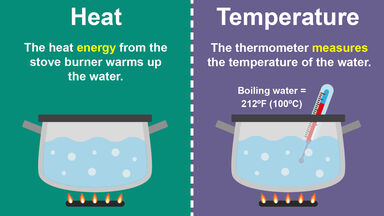
. Heat is fundamentally a change in energy during some process. The fundamental difference between heat and temperature is slight but significant heat is the overall energy of the molecular motion whereas temperature is the average energy of the molecular. To understand it clearly in scientific terms heat is the overall energy of the motion of the molecules while temperature is the average energy of the molecular motion.
If we add 1 Joule of energy to one gram of air at 20 o C it will raise the temperature to about 21 o C. The change in internal energy as a result of temperature difference between the system and the surroundings is reffered to as heat. The temperature of an object depends on the state of motion of its particles.
The temperature is related to how the entropy changes when you change the energy a little bit. For example if you put a room-temperature metal spoon into a hot liquid like soup or hot chocolate the metal gets hotter. The magnitude of these temperature changes depends on the mass and heat capacities of the two objects.
Often the concepts of heat and temperature are thought to be the same but they are not Heat and Temperature para. Yet both the terms imply a very different meaning. On the other hand when work is done on a system the capacity of the system to do work increases in other words the energy of the system is increased.
However it is usually expressed in terms of temperature using the formula. The relationship between pressure and temperature of a gas is stated by Gay-Lussacs pressure temperature law. The amount of heat lost by the warmer object is equal to the amount of heat gained by the cooler object.
There exist a fine line which demarcates heat from temperature in the sense that heat is thought of as a form of energy but the temperature is a measure of energy. The warmer objects temperature will drop and the cooler objects temperature will rise until they reach the same temperature. It relates to the average kinetic energy of microscopic.
T is not energy. When they are heated they move faster and when they are cooled they move slower. HEAT TEMPERATURE AND CONDUCTION All things are made of atoms and molecules which are always in motion.
Therefore as the pressure of a particular system goes up the temperature of that system also goes up and. The main difference between heat and temperature is heat is a form of energy whereas temperature is the measure of energy. Revise the relationship between energy specific heat capacity mass and change in temperature as part of National 5 Physics.
What youre referring to is internal energy not heat. Energy is an extensive quantity that does depend on the amount of matter involved. When system does work the energy of the system is reduced.
Specifically its the change in internal energy which is not attributed to. Difference between heat temperature and thermal energy. The proximity results in collisions with less force and lower air pressure.
Heat is the total energy of the motion of the molecules inside the object or particle whereas Temperature is merely a measure of this energyThe relationship could be the more heated an object is there higher the temperature the object will have. The quantitative relationship between heat transfer and temperature change contains all three factors. More heat usually means a higher temperature.
Heat flows from higher temperature object to lower temperature object amount of heat lost amount of heat gained warmer temperature will drop cooler will rise until they are the same temperature magnitude of these temperature changes depends. The symbol c stands for specific heat and depends on the material and phase. This law states that the pressure P of a fixed mass of gas held at a constant volume is directionally proportional to its Kelvin temperature T.
Thermal energy is the total of kinetic energyTemperature is a measure of average heatHeat is the average of kinetic energy. Thats not at all what heat or temperature is. Air density plays a role in the correlation between temperature and pressure because warmer air is less dense than cool air allowing molecules to have more space to collide with greater force.
If we add 1 Joule of energy to one gram of liquid mercury at 20 o C it will raise the temperature to about 27 o C. This analogy bears absolutely no resemblance to the truth. Temperature and thermal energy both are physical properties both are the thermodynamic state nature of an object.
If we add a Joule of energy to one gram of liquid water at 20 o C it will raise the temperature to about 202 o C. Particles present in a substance moves at different speed particles moving at a slower speed has less kinetic energy cooler in temperature whereas particles moving at a faster speed posses. This is not true at all.
For simple systems like the ideal gas you have a simple relationship between the temperature and the average kinetic energy of each gas molecule. QmcΔT Q mc Δ T where Q is the symbol for heat transfer m is the mass of the substance and ΔT is the change in temperature. Heat and Temperature Heat is often described by the average individual as being the change in temperature from hot to cold.
Temperature is an intensive quantity that does not depend on the mass of material that you have. It is the total amount of energy both kinetic and potential possessed by the molecules in a piece of matter. Heat and temperature are related and often confused.
Temperature of a body increases if it only gains heat and temperature decreases if a body. K C_v dT dx where k is thermal conductivity Cv is the specific heat capacity dT dx is the temperature gradient across the material and x is the physical dimension over which the temperature difference occurs. Heat is the total size of the pizza temperature is the average size of the slices.
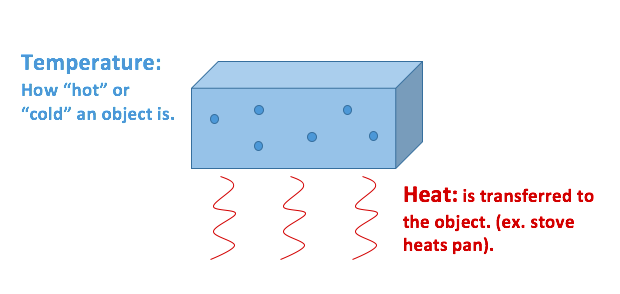
The easiest way to picture the difference between the temperature and energy of an object is in terms of a particles model.
Difference Between Heat And Thermal Energy Difference Between
No comments for "Explain the Difference and the Relationship Between Heat and Temperature"
Post a Comment